Abstract
Background In patients with acute myeloid leukemia (AML) in complete remission (CR), the presence of measurable residual disease (MRD) detected by next generation sequencing (NGS) prior to allogeneic blood or bone marrow hematopoietic stem cell transplant (alloHCT) is associated with an increased risk of post-transplant relapse. The use of myeloablative conditioning in patients with NGS-based MRD can mitigate this increased risk; however, the prognostic value of MRD testing for specific leukemia-associated mutations is not yet known. 20% of AMLs bear mutations in the isocitrate dehydrogenase genes (IDH1/2), rendering them sensitive to IDH inhibitors. It is not known whether the presence of pre- or post-transplant mutant IDH MRD has prognostic significance, and if so, whether treatment with an IDH inhibitor would mitigate this risk. In this study, patients with IDH-mutated AML undergoing alloHCT were tested for the presence of IDH MRD pre- and post-transplant to determine the prognostic value of IDH MRD.
Methods The study included consecutive patients with IDH-mutated AML who underwent non-myeloablative alloHCT using post-transplant cyclophosphamide-based graft versus host disease prophylaxis at the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center between 2015 and 2020 and who had pre- and post-transplant bone marrow samples available for IDH MRD testing. Baseline and relapse DNA samples were sequenced using a 75 gene panel based on anchored-multiplex PCR to identify co-mutations. Pre- and post-alloHCT DNA samples were assessed for presence of IDH mutations by digital droplet PCR (ddPCR) or error-corrected targeted next generation sequencing (ecNGS), depending on DNA availability. Assays were extensively validated, and mutations were detectable at variant allele frequencies (VAF) down to 0.01-0.05% and 0.05-0.1% for the ddPCR and ecNGS assays respectively.
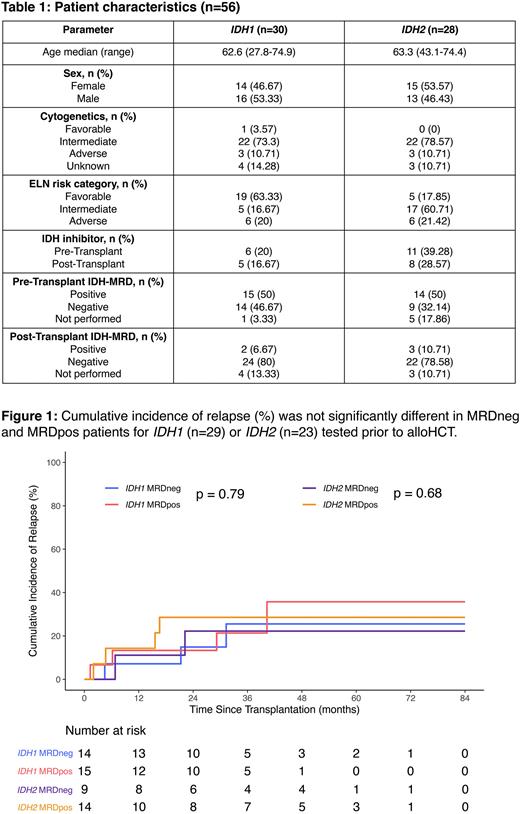
Results Of 56 patients with baseline DNA samples available, 28 and 26 had mutations in IDH1 and IDH2 respectively, while 2 patients had mutations in both. Complete remission was achieved in 55 patients prior to transplant. Patient characteristics are shown in Table 1. DNMT3A, NPM1, SRSF2, FLT3 and ASXL1 were frequently co-mutated with IDH1 while DNMT3A, BCOR, SRSF2, NPM1 and FLT3 were observed with IDH2. ddPCR/ecNGS could be performed on 50 pre-alloHCT samples, of which 28 (56%) were MRD positive for mutations in either or both IDH genes (VAF median: 4.13%, range: 0.15%-49%). The overall survival (OS) and cumulative incidence of relapse (CIR) were not significantly different between the MRD positive and negative groups for IDH1 (OS: 87% vs 81% at 3 years, p=0.66, Relapse: 21% vs 26% at 3 years, p=0.79) or IDH2 (OS: 63% vs 67% at 3 years, p=0.55, Relapse: 29% vs 22% at 3 years, p=0.68) (Figure 1). At the post-alloHCT timepoint, 5/48 (10%) samples tested were MRD positive. Two of these patients relapsed and died while the remaining three maintained remission. The original IDH mutation was revealed at relapse in 9/10 (90%) patients who had DNA available for sequencing. Additional mutations with VAFs ranging from 2-33% were present in 9 relapse samples.
Conclusion In this cohort of patients with IDH-mutated AML who underwent non-myeloablative alloHCT, the presence of pre- or post-transplant IDH MRD was not prognostic of leukemia relapse. This analysis is limited by the small number of relapses within each group and the substantial rate of pre- or post-transplant use of IDH inhibitors.
Disclosures
Hourigan:TwinStrand Biosciences: Other; Qiagen: Other; Archer Diagnostics: Other; Mission Bio: Other; Sellas Life Sciences (Inst): Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal